Methods
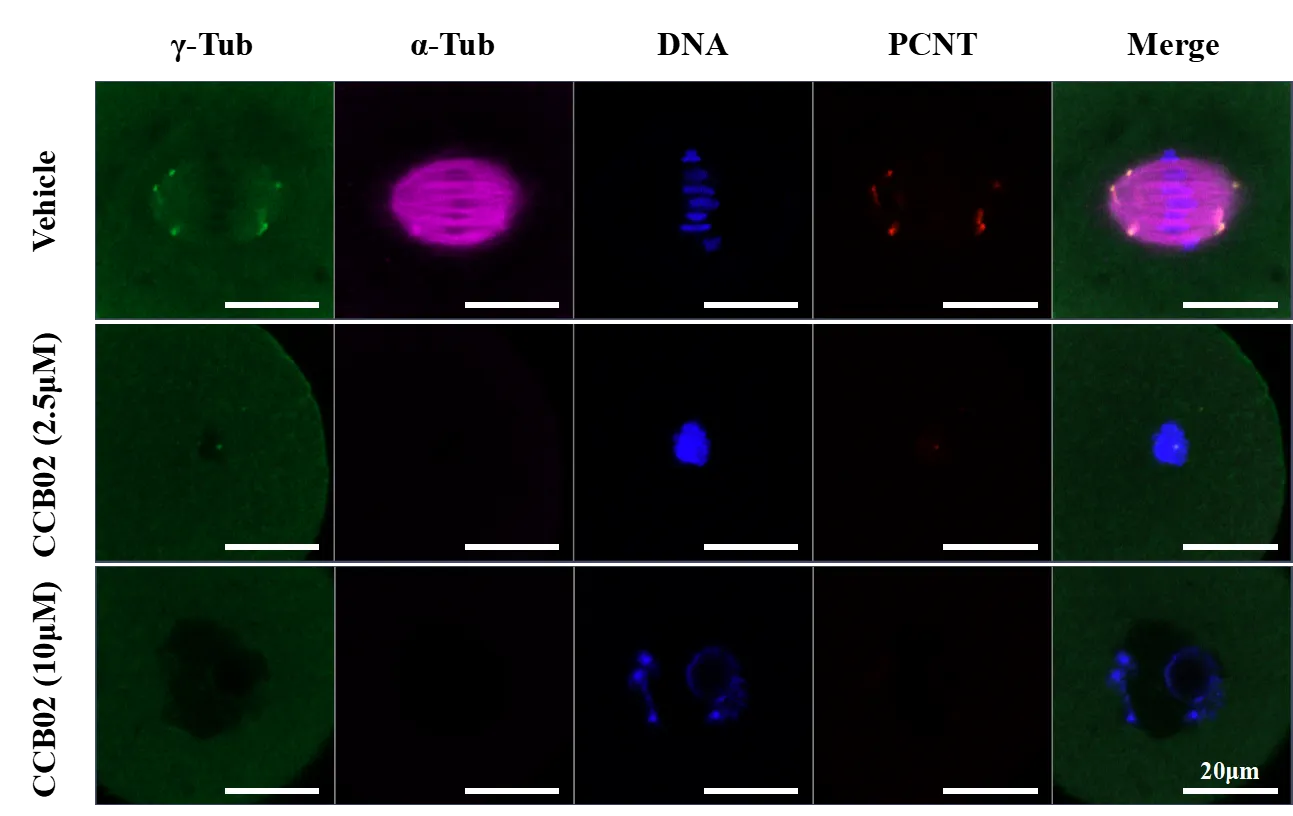
GV-stage mouse oocytes were isolated and treated with gradient concentrations of CCB02 (0 mM, 2.5 mM, 5 mM, and 10 mM) for 7 hours, followed by fixation, immunofluorescence staining, and confocal microscopy imaging.
Wuhan University
National Institute of Biological Sciences
Jul 2025 - Aug 2025
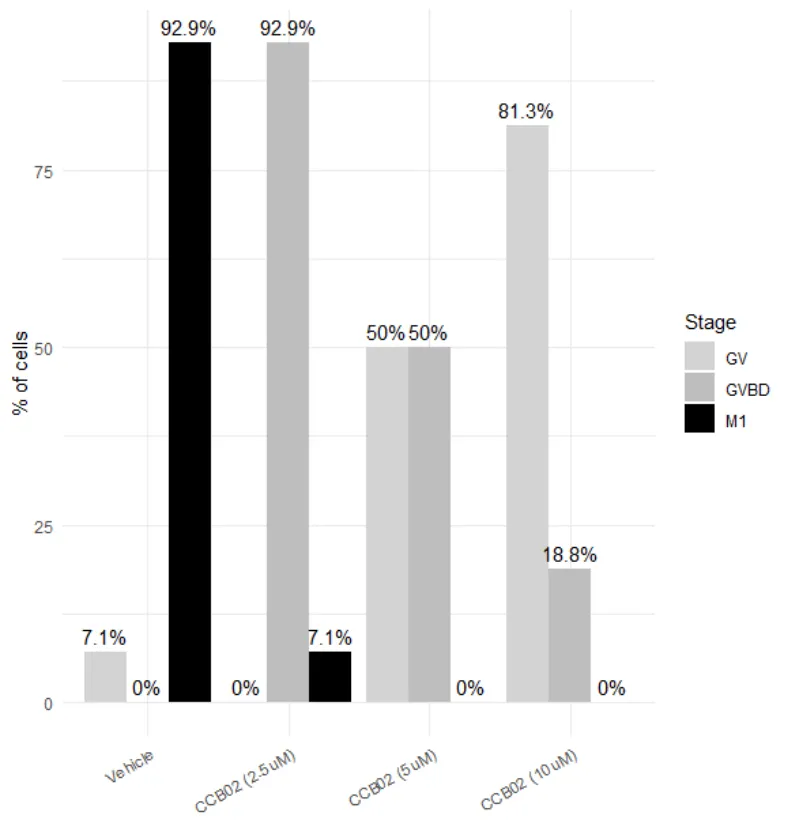
CCB02 binds the CPAP-binding site on tubulin, disrupting centrosome function in somatic cells. Mouse oocyte meiosis, which is centrosome-independent, was treated with increasing CCB02 concentrations (0–10 mM) for 7 hours. Spindle assembly was impaired at all doses, and higher concentrations delayed meiotic progression, increasing the proportion of cells at earlier stages. These results demonstrate a dose-dependent inhibitory effect of CCB02 on oocyte meiosis, providing insights into spindle assembly regulation during oogenesis.
CCB02 was identified and optimized to selectively bind to the CPAP-binding site on tubulin, thereby competitively inhibiting the interaction between CPAP and tubulin, allowing CPAP to recruit greater amounts of its other interacting proteins, such as pericentriolar material (PCM), to interphase centrosomes. This disrupts normal centrosome clustering, activates the spindle assembly checkpoint (SAC), and ultimately prevents cell division.
However, mouse oocyte meiosis relies on a centrosome-independent mechanism of spindle assembly, in which CPAP does not localize to the spindle. To better understand this process, we investigated the effect of CCB02 during mouse oocyte meiosis. Our results showed that mouse oocyte meiosis was inhibited by CCB02 in a dose-dependent manner, providing new insights into the regulation of spindle assembly during oogenesis.
GV-stage mouse oocytes were isolated and treated with gradient concentrations of CCB02 (0 mM, 2.5 mM, 5 mM, and 10 mM) for 7 hours, followed by fixation, immunofluorescence staining, and confocal microscopy imaging.

Oocytes treated with a low concentration of CCB02 (2.5 mM) for 7 hours progressed to GVBD stage, whereas those treated with a high concentration (10 mM) remained at the GV stage; in both conditions, spindle assembly was defective.
Increasing CCB02 concentration slowed oocyte development, raising the proportion of cells at earlier meiotic stages. Our findings complement previous work by demonstrating the effects of CCB02 in oocytes that undergo centrosome-independent spindle assembly, highlighting its relevance to meiotic progression during oogenesis.